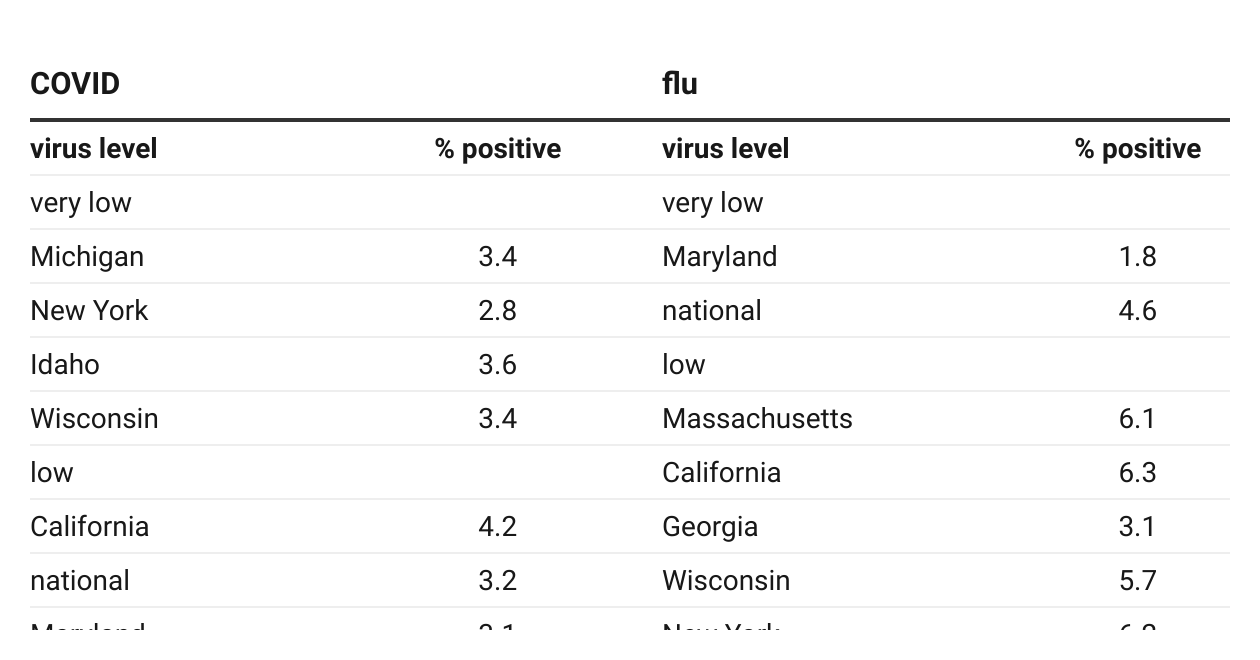
All COVID and flu metrics down, but deaths persist. COVID virus levels in wastewater are low nationally and for the third week, low in all regions. The 3.2% positive rate in clinical testing is also below November’s when the winter surge began (Table 1 derived from CDC data). However, although the week’s deaths accounting for 0.6% of all COD is a low for the year and below the rate at the surge’s start, serious COVID turning deadly persists and is higher than for flu for the fifth week, totaling 1585 v. 1127 for the period (CDC).
Flu A in wastewater is now very low nationally. In the latest week, flu B accounts for 70% of the virus in clinical testing (CDC). The 4.6% (A + B) positive test rate is still higher than in November. The week’s death rate is down to 0.2% of all COD, but the season’s pediatric toll of 216 is the highest in 15 years.
No bird flu was reported in humans.
Vaccines
There are no data to look at for new COVID vaccines this week, but there’s a lot of news about the future of vaccines in the US.
• Fulsome implementation of policies driven by Kennedy’s “understanding” of vaccines – that they are neither safe nor effective and in some cases never worked, and that the data are lacking to support their use. With decisions due on their approvals and policies for the fall, the future of vaccines emerged rapidly last week albeit with some clarity lacking. Thus, the two most prominent:
• depending on the definition of “new” vaccines, the years-old Novavax protein shot will need additional clinical trials to get a license as could any fall updates of the Pfizer and Moderna mRNA jabs
• placebo testing will be included in all clinical trials
How will these goals be implemented?
• Additional clinical trials for Novavax FDA didn’t meet the deadline to grant a license for the protein-based vaccine a month ago apparently due to the agency asking for additional data to be collected after approval (AP). However, FDA indicated last week that the current vaccine under consideration, the 2024-2025 update for the Omicron JN.1 subvariant, is considered to be a new vaccine that needs to undergo a clinical trial for safety.
Novavax is currently under Emergency Use Authorization for people 12 years and older. Without a license, the EUA could be pulled given that the Public Health Emergency for COVID officially ended almost two years ago. Commissioner Makary claimed the FDA is “prioritizing the Gold Standard of Science” and “radical transparency” in the approval process (although it’s unclear what these phrases mean).
Also in question is whether this new standard will be applied to any updates of the Pfizer and Moderna mRNAs (see below). Updated boosters of the original vaccines undergo small trials to confirm that the antibody response is not inferior to that with the current shots. In addition, safety continues to be monitored throughout the authorized use or license just as have the original 2020 and subsequent updated vaccines.
It's clear that HHS’ Kennedy is behind the new standard. Among other claims, he believes single-antigen vaccines like Novavax don’t work for respiratory diseases.
• May 22 VRBPAC on fall vaccines? will be much anticipated for clarifications about the future of vaccines in general. Although CNN reported an unnamed source revealing the date, there's no notice on the FDA calendar as of early Sunday night. An(other) unnamed source previously suggested the agency might not even consider updated shots for this fall.
FDA’s Makary said the previous and current boosters lacked “good data,” parroting Kennedy’s show-me-the-data (CBS News). He questioned the benefit of boosters in an environment of population immunity acquired by vaccination and/or infection, which doesn’t acknowledge that updated shots have consistently demonstrated supplemental effectiveness against new variants, especially in providing protection against serious COVID in older populations.
Nonetheless, there’s sure to be many questions from the committee about the multiple reports pointing to the revamping of how current and "new" vaccines will be authorized or licensed, which could include additional clinical trials to test updated boosters. This would go beyond the holdup of the license for Novavax and could delay the timely rollout of seasonal shots.
A selection of the variant to target for 2025-2026 might have to wait for what drives a summer surge anyway. Omicron seems to have slowed in changing its subvariants (https://covidandvaccineupdate.substack.com/p/omicron-wanes-with-flu-and-measles).
Moderna is also due an FDA decision by May 31 on its mRNA-1283 vaccine for COVID that targets a truncated portion of the S(pike) protein (CNN, https://covidandvaccineupdate.substack.com/p/covid-and-flu-still-nothing-to-sneeze).
• Placebo testing According to an HHS notice, “All new vaccines will undergo safety testing in placebo-controlled trials … - a radical departure from past practices.” Except that’s how the original COVID vaccines were tested as are other new vaccines.
The new HHS wants to use inert substances such as saline (physiological amounts of salt in water) for placebos. If the agency considers updated boosters to be new and require additional trials, using a saline placebo would be medically unethical. When a similar vaccine already shown to be safe and effective, such as the previous version, is available, it is unacceptable to withhold it from participants in the control group who could be at risk for serious illness (WHO). In the original trials of the COVID vaccines, participants in the control group were offered to switch from the placebo to the candidate vaccine as soon as it was statistically determined to be efficacious.
Besides the medical ethics, the aim is to measure the added benefit of the booster against any increased safety risk compared to the previous similar jab that should be used as the control.
In chasing an ever-changing virus, the mRNA vaccines have the significant advantage of adapting with relatively easy, minor changes. This head start is lost if additional time-consuming clinical trials are mandated in the short sprint to stay ahead.
© 2025 Henry A. Choy. All rights reserved.